3.1 Creation of Complex Elements
-
1 Opener
-
5 Activities
-
5 Videos
-
2 Articles
-
1 Closer
Introduction
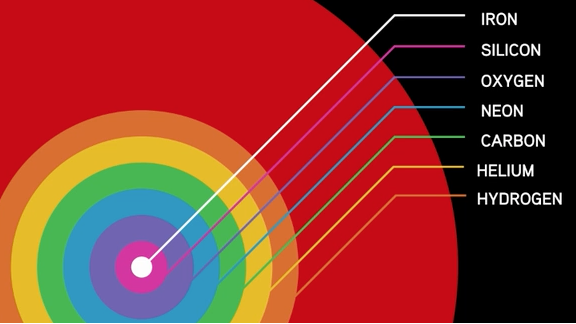
With the birth of stars, new sources of light and energy emerged all over the Universe. They burned hydrogen to create helium. Helium was used to create carbon. Neon, oxygen, silicon, and iron were also created during the lives of stars. However, it is once these stars started running out of fuel that things really got interesting. It is in the massive explosions that resulted from certain stars running out of fuel that all of the elements of the periodic table were created. Without the death of stars, none of our world would exist today.
More about this lesson
- Describe what happens in the death of a star, and how that results in the creation of chemical elements.
- Explain why some elements are formed during the life of a star, like helium and carbon and others, like silver, are not.
- Describe how the death of a star is connected to your immediate surroundings.
Is It In There?
Preparation


Purpose
This lesson emphasizes the connection between the chemical elements created in dying stars and the world around us today. Looking at a variety of everyday objects, you will guess whether certain elements are found in these objects.
Process
If you want to get a real sense of the connection between our daily lives and dying stars, you don’t need to look very far. Chemical elements formed in the dramatic explosions of dying stars are essential to products and systems we use every day. In this brief activity, you will work in groups to determine which chemical elements are found in the following: a cell phone, a car, the Golden Gate Bridge, and a blueberry muffin. With your group, complete the Is It in There? Worksheet. You have only 5 minutes. Once the class has completed their sheets, be prepared to explain which elements you felt were in one of the items. If your group is not presenting, keep track of which elements your group believes are in that same item, and be ready to explain if you came to a different conclusion than the other group about what elements should be included.
Narratives and Thresholds – New Chemical Elements
Preparation


MP4 / 2:50
You will need a copy of the Thresholds Graphic you worked on in earlier Narrative and Thresholds activities. If you skipped the previous activities, you can use the Threshold Graphic included in the PDF for this activity.
Purpose
Big History Project research has shown the importance of both teachers and students deeply understanding the driving course narrative, which is built around eight thresholds of increasing complexity. Internalizing the narrative of the BHP story will give you a framework in which to place the content you learn throughout the course. This activity continues the series of repeated tasks to help build knowledge of the thresholds and the overall Big History Story.
Practices
Reading, scale
While you aren’t engaging in formal reading of texts as part of this activity, you do analyze an image, which can be considered a form of reading. Additionally, you are pressed to think about scale in this activity by trying to think about the origins of gold and how gold can be traced through the threshold. Instead of a zoomed-out origin story such as the Big History story, you will start to think about zooming in on things in our world to figure out those origin stories, which will help prepare you for your Little Big History Project.
Process
Take out the BHP Thresholds Graphic and read your summaries for Thresholds 1 and 2. Then look at the graphic for Threshold 3: New Chemical Elements. Think about the following questions to connect your ideas to the third threshold:
- How has what you’ve learned since writing about Thresholds 1 and 2 supported, extended, or challenged your thinking about those two thresholds?
- What does the image tell you about new chemical elements? What do you think are some of the key ideas of this threshold and why do you think that?
Next, watch Threshold 3: New Chemical Elements. Think about how this threshold is connected to previous thresholds—and how it might link to what is to come. When you’re done, write a tweet (288 characters or less) and add it to your Threshold Graphic.
One of the new chemical elements described in this threshold is gold. Gold is not only important culturally (we see it frequently in our daily lives), but it was foundational in forming our world. Discuss the following questions with your class:
- Where do you see gold today?
- How does gold relate to the thresholds you’ve already encountered? Can you trace its origins?
- How might gold relate to the thresholds you’ve yet to study?
As you probably remember from earlier in the course, you can look at origin stories on a grand scale but also on a smaller scale. For example, you learned in Unit 1 that each person has their own origin story. Really, everything we encounter in the world has an origin story, whether it’s a person, an animal, or even an object! Everything started from somewhere, including gold.
Vocab Tracking
Preparation

Purpose
This repeated activity should help you become familiar with a process for understanding unfamiliar words anytime you encounter them in the course.
Process
Take out your vocab tracker and be sure to record new and unfamiliar words on it according to your teacher’s instructions.
Threshold 3 – New Chemical Elements
Vocab Terms:
- collapse
- element
- fuse
- nucleus
- supernova
Summary
It is only in the death of a dying star that we see temperatures high enough to form the chemical elements found all around us today.
Threshold 3: New Chemical Elements (2:50)
Key Ideas
Purpose
This short introduction to Threshold 3, New Chemical Elements, quickly introduces the threshold, setting the stage for the rest of the concepts of the unit.
Process
Preview
Stars begin to light up all over the Universe. They’re not light forever though. Eventually, they will run out of fuel. When small stars run out of fuel, they just fizzle out. Imagine a campfire when there’s no more wood to burn. Bigger, denser stars burn hotter. When they run out of fuel, there are massive explosions that result in new chemical elements.
Key Ideas—Factual
Think about the following questions as you watch the video:
- What are the Goldilocks Conditions for new chemical elements?
- What is a supernova?
Thinking Conceptually
How many of the objects within the space immediately around you are born out of the death of star?
Causation – Star Formation Part 2
Preparation

MP4 / 9:10
Purpose
This activity revisits the causal map you created in Causation – Star Formation Part 1. This time, you’ll add details from Threshold 3, which will complicate your causal maps and deepen your understanding of how to use this analytical tool. Doing so should help you understand how new chemical elements are formed, and should also help you see how Thresholds 1, 2, and 3 are connected. This activity helps drive home how we use cause and effect as a tool for studying history. It also reinforces the idea that causes and effects are often two sides of the same coin; it’s the perspective you take that determines whether something is a cause or an effect.
Process
In this activity, you will build upon your star-formation causal maps, adding new effects related to Threshold 3: New Chemical Elements. You will identify the short-term, intermediate-term, and long-term causes and effects of star formation.
Identifying Causes and Effects
- Watch the video, What Did Stars Give Us? Think about star formation and the effects of star formation as you watch.
- Take out the Causes Cards along with the Causation Tool, which is included in the Causation – Star Formation Part 2 worksheet. Divide the cards into long term, intermediate term, and short term (you can place them on the relevant sections of the tool if you want). You may also choose to place some of the cards in the Effects box at the bottom of the tool. Don’t forget to put the event in the middle, and make sure to label the triggering event with a star (*). Be prepared to discuss your placement with your class.
- Now, you’re going to create another causal map of star formation, but this one will be a bit more complicated than the last one.
- Once you’ve made your map, label each of the squares with either a “C” (for causes) or an “E” (for effects).
- Think about your map’s connection to Threshold 1 (The Big Bang). Our map starts with gravity pulling atoms of helium and hydrogen together in dense clouds. But the chain of causes didn’t start here.
- What were the causes that brought us to this point?
- How does changing the perspective by extending the timeframe make things seem more or less important than they did before we expanded our causal maps?
What Did Stars Give Us?
Vocab Terms:
- element
- fuel
- nucleus
- periodic table
- proton
Summary
Iron, gold, copper, and nickel can be created only in the incredible heat of massive stars, or in the dynamic explosion that happens as they die. These elements play an important role in the daily lives of many people. As we continue the story, we’ll learn more about the properties of these elements.
What Did Stars Give Us? (9:10)
Key Ideas
Purpose
In the death of a star, incredibly high temperatures result in the formation of every element of the periodic table. These elements, floating in space in the aftermath, eventually find their way into new stars, planets and all of the life here on Earth.
Process
Preview
It takes a lot of heat to fuse together two atoms. The larger the atom, the more heat is required. While stars are incredibly hot, they are nowhere near hot enough to form anything more than a few basic atoms. Once a star runs out of fuel, it will either fizzle out or explode. These explosions generate staggering temperatures, hotter than the star itself. In a few seconds, all of the elements of the periodic table are reached.
Key Ideas—Factual
Think about the following questions as you watch the video:
- When you add heat (or energy) to a pot of cold water, what happens? Does the pot stay still? What does this tell us about when we add energy to hydrogen atoms?
- Why do some stars simply fade away, while others eventually start fusing helium into carbon?
- Will all stars eventually be able to generate iron?
- Do elements only exist in large amounts on Earth?
Thinking Conceptually
How does the fact that valuable resources like copper and gold can only be formed in the death of stars influence the way we think about these elements today?
Crash Course Big History: Why Star Stuff Matters
Vocab Terms:
- carbon
- carbon dioxide
- coal
- complexity
- oxygen
Summary
While the history of human life is only a fraction of the 13.8 billion years our Universe has existed, the elements that accelerated complexity matter a great deal. Carbon in particular is a foundational element that got us here, and as carbon-based life forms we’d be nothing without it. Further, our modern world is powered mostly by the gifts carbon left us millions of years ago.
Crash Course Big History: Why Star Stuff Matters (11:03)
Key Ideas
Purpose
This video will help you gain an understanding of the element carbon—how it came into existence, then how it led the way for the creation of other elements and, ultimately, life. As you dive into the significance of carbon in our past, present, and future, you’ll begin to see ways that this knowledge can be applied to other discussions.
Process
Preview
In addition to knowing the history of events, it is essential to ask why they matter. Our ability to grasp how the Universe grew and changed over nearly 14 billion years—and how it may eventually expire—gives meaning to our place in it. Theories of cosmic inflation, multiverses, and cosmic simulation may boggle the mind, but they are currently our most educated guesses.
Key Ideas—Factual
Think about the following questions as you watch the video:
- What was the Universe like before carbon existed, and what was it like after?
- What makes carbon so important on Earth?
- How did the flexibility of carbon lead to the first life forms?
- How is charcoal responsible for “the next rise of complexity”?
- Why is it possible that carbon may ultimately be our downfall?
Thinking Conceptually
How do historians decide what really matters? Although carbon and its role in life is obviously the concern of scientists, why do historians also care so much about it?
The Evolving Star: Subrahmanyan Chandrasekhar – Graphic Biography
Preparation

Summary
Subrahmanyan Chandrasekhar (1910–1995) was born in Punjab in British India. He displayed remarkable scientific genius at a young age, publishing his first paper at 19. Over the course of his life, he made many discoveries in physics. His most famous was the idea that the life and death of stars followed paths in accordance with their mass. This mass was called the Chandrasekhar Limit, which helped astronomers predict if a star would end as a white dwarf, a black hole, or a neutron star. He won the Nobel Prize in Physics in 1983 for this research.
Purpose
Understanding the lifecycle of stars helps us figure out the evolution of the Universe up to this point and estimate what will happen in the future. This graphic biography presents the journey Subrahmanyan Chandrasekhar’s life took from a curious young boy to the recipient of the Nobel Prize in Physics. His work explored important ideas in physics. Today, his ideas and findings are used by countless scientists around the world. As we develop better understandings of the Universe around us, Chandrasekhar’s findings will continue to inform our collective learning.
Process
Read 1: Observe
As you read this graphic biography for the first time, review the Read 1: Observe section of your Three Close Reads for Graphic Bios Tool. Be sure to record one question in the thought bubble on the top-right. You don’t need to write anything else down. However, if you’d like to record your observations, feel free to do so on scrap paper.
Read 2: Understand
On the tool, summarize the main idea of the comic and provide two pieces of evidence that helped you understand the creator’s main idea. You can do this only in writing or you can get creative with some art. Some of the evidence you find may come in the form of text (words). But other evidence will come in the form of art (images). You should read the text looking for unfamiliar vocabulary words, the main idea, and key supporting details. You should also spend some time looking at the images and the way in which the page is designed. By the end of the second close read, you should be able to answer the following questions:
- What issues did Chandra face when he presented his calculations about the death of stars?
- What was Chandra’s experience at the University of Chicago like?
- What was Chandra’s most important contribution to collective learning?
- How has the artist designed the images in this comic to help you know in which order to read the text?
- Looking at just the images, what do you think is the theme of this comic?
Evaluating and Corroborating
Read 3: Connect
In this read, you should use the graphic biography as evidence to support, extend, or challenge claims made in this unit of the course. On the bottom of the tool, record what you learned about this person’s life and how it relates to what you’re learning.
- What does Chandra’s experience sharing his ideas about stars teach you about the process of collective learning? How did his theory become accepted?
To Be Continued…
On the second page of the tool, your teacher might ask you to extend the graphic biography to a second page. This is where you can draw and write what you think might come next. Here, you can become a co-creator of this graphic biography!
“A Little Big History of Silver”
Vocab Terms:
- currency
- element
- hydrogen
- mine
- scarce
Preparation

Summary
Silver has a story that is born out of the stars and plays an important role in our lives today. Its value and scarcity lead to trade and in many cases, battles. This is the first Little Big History you have seen in the course. By the end of the year, you will be writing your own Little Big History.
Purpose
A Little Big History is a story told about an object or an idea from the perspective of Big History. These histories include aspects of multiple thresholds and the perspective of multiple disciplines. At the end of the course, you will write your own Little Big History about an object or idea you choose.
Process
Skimming for Gist
This article contains a lot of information about silver. However, not all informative articles are interesting. As you read this article pay attention to the writing. Did the author do anything to interest the reader?
Understanding Content
By the end of the second close read, you should be able to answer the following questions about silver:
- What characteristics of silver make it valuable as a mineral?
- Name a few places where silver was found on Earth? Where was it scarce?
- Describe two uses of silver.
- Describe the role of silver in trade along the Silk Road.
Thinking Conceptually
At the end of the third close read, respond to this question: Think about how the properties and location of chemical elements such as silver impact our lives today. What would be different if silver were as plentiful as carbon? What would it mean if silver were only found in one place on the planet?
Superhero Element
Preparation


Purpose
The creation of new chemical elements is quite possibly one of the most important thresholds in the story of us. Everything that we know of, including Earth, our bodies, the food we eat, the homes we live in, and the phones we use every day, are made of the chemical elements created in the death of stars. This activity will help you see how these elements are important in our everyday lives and how the reactions between elements create even more complexity.
Process
Choose an element from the list on the worksheet and create a superhero profile. On the first page, you will design a superhero and a tagline that embodies the characteristics of your element including the atomic number, symbol, and name. On the second page, you will tell the origin story of your element. Be creative when telling your element’s story, which may be told in any format you wish, such as a myth or cartoon. A superhero sidekick or nemesis may also be included but you must choose an element that either combines well or has a violent reaction with your chosen element. A rubric for this activity is included with the Superhero Element Worksheet.
